STUDY ON DIELS–ALDER REACTION OF NITROSOALKENES
Keywords:
indoles, nitrosoalkenes, oximes, cycloaddition, Diels–Alder reactionAbstract
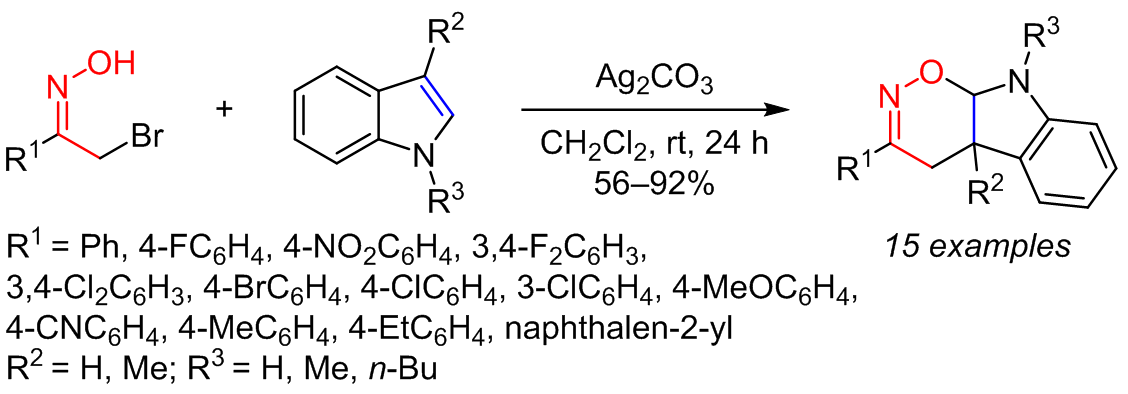
The reactions of nitrosoalkenes, generated in situ from the corresponding α-bromooxime upon the action of a base, with electron-rich olefins were used to synthesize a series of heterocyclic compounds containing 1,2-oxazines, the expected Diels–Alder cycloadducts. The structural features of the obtained heterocyclic compounds were determined based on NMR spectra, HRMS, and X-ray structural analysis.
Downloads
Published
2024-01-10
Issue
Section
Original Papers
