HIGHLY DIASTEREOSELECTIVE SYNTHESIS OF PYRIDINIUM-SUBSTITUTED PIPERIDIN-2-ONES FROM PYRIDINIUM YLIDES, ALDEHYDES, MICHAEL ACCEPTORS, AND AMMONIUM ACETATE
Keywords:
four-component reaction, 2-piperidinones, pyridinium salts, pyridinium ylides, ammonium acetate, stereoselectivityAbstract
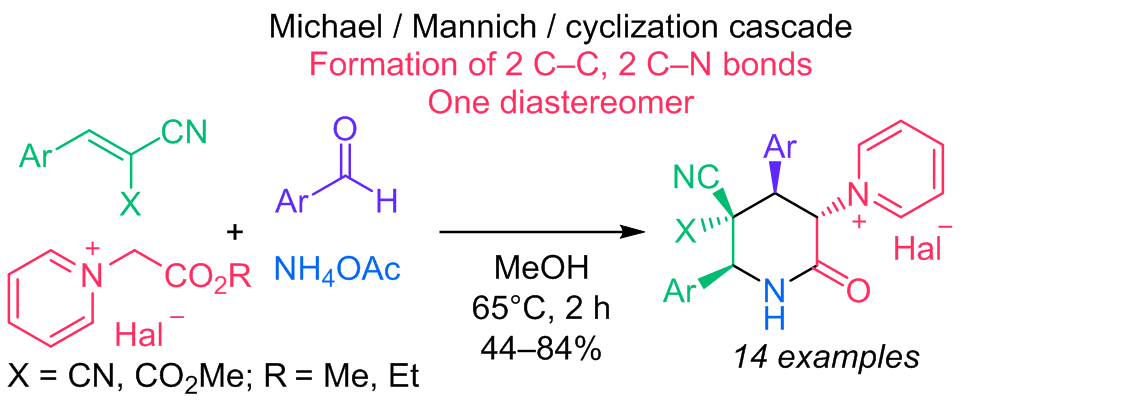
A novel four-component diastereoselective synthesis of piperidin-2-one salts containing a quaternized pyridine unit is reported. The Michael–Mannich cascade was conducted using Michael acceptors, pyridinium ylides, aromatic aldehydes, and ammonium acetate in methanol. It is a convenient approach to the synthesis of 1-((3SR,4RS,6SR)-4,6-diaryl-5,5-dicyano-2-oxopiperidin-3-yl)pyridin-1-ium halides with three stereocenters in 48–84% yield or 1-[(3SR,4RS,5RS,6SR)-4,6-diaryl-5-cyano-5-(methoxycarbonyl)-2-oxopiperidin-3-yl]pyridin-1-ium halides with four stereocenters in 44–74%. This reaction is highly stereoselective. Only one diastereomer was formed. Ammonium acetate plays a dual role, acting as a base and as a nitrogen source. Structures of the synthesized compounds were confirmed by 1H, 13C NMR, IR, and mass spectra. The formation of a single diastereomer was confirmed by singe crystal X-ray diffraction studies. Products were obtained by simple filtration, and other purification methods as column chromatography were not necessary.