THE REACTION OF 4-HYDROXY-6<i>H</i>-1,3-OXAZIN-6-ONES WITH AMIDINES – A ROUTE TO ACCESS NEW 1,3,5-TRIAZINE DERIVATIVES
Keywords:
acetamidine, benzamidine, 4-hydroxy-6H-1,3-oxazin-6-ones, 1,3,5-triazines, recyclizationAbstract
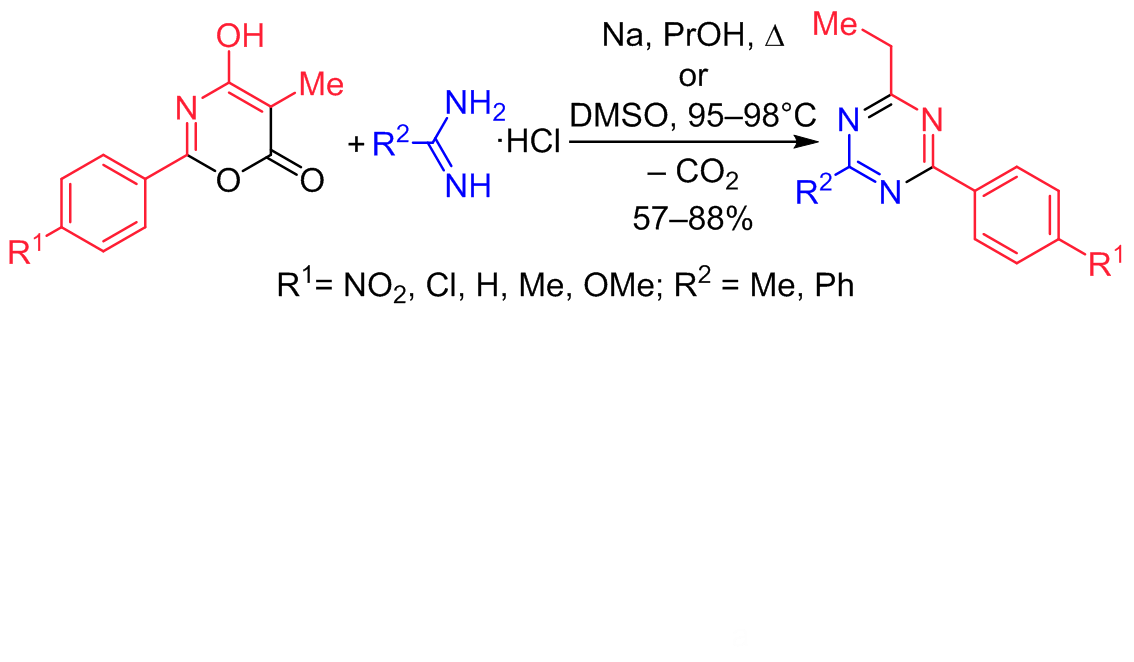
The reaction of 2-aryl-5-methyl-4-hydroxy-6H-1,3-oxazin-6-ones with 1,3-binucleophilic reagents acetamidine and benzamidine was studied. It was established that in n-propanol under reflux in the presence of sodium n-propoxide or in DMSO, the predominant reaction products were 1,3,5-triazine derivatives. It was shown that the reaction time and the yield of the target product were significantly influenced by the choice of the solvent, the nucleophilicity of the amidine, and the electronic structure of 4 hydroxy-6H-1,3-oxazin-6one.
Downloads
Additional Files
Published
2024-03-26
Issue
Section
Original Papers
