A REACTION OF 2-CARBONYL-SUBSTITUTED 1<i>H</i>-BENZO[<i>f</i>]CHROMENES WITH <i>N</i>-ARYLAMIDES OF CYANOACETIC ACID
Keywords:
active methylene nitriles, N-aryl-2-cyanoacetamides, 1H-benzo[f]chromenes, 2-pyridones, Dimroth rearrangement, Michael reaction, Thorpe–Ziegler cyclizationAbstract
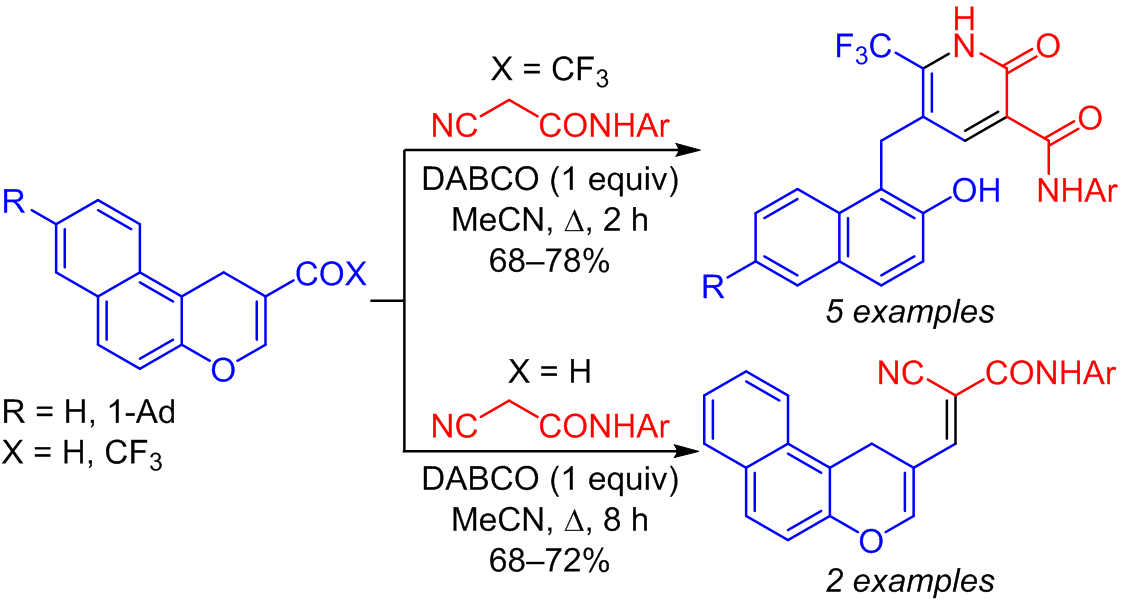
A reaction of 2-trifluoroacetyl-1H-benzo[f]chromenes with N-arylamides of cyanoacetic acid led to the synthesis of a series of 2-oxo-6trifluoromethylpyridine-3-carboxamides as products of a cascade of the carbo-Michael reaction, Thorpe–Ziegler cyclization, and Dimroth rearrangement. In the case of 1H-benzo[f]chromene-2-carbaldehydes, the corresponding Knoevenagel adducts were isolated.
Downloads
Additional Files
Published
2024-05-28
Issue
Section
Original Papers
