A REACTION OF AROYL(HETEROAROYL)PYRUVIC ACID ESTERS WITH 3,3-DIAMINOACRYLONITRILES: A NEW METHOD FOR THE SYNTHESIS OF PYRROLES WITH CONJUGATED EXOCYCLIC DOUBLE BONDS
Keywords:
3,3-diaminoacrylonitriles, methyl pyruvates, pyrroles, pyrrolones, C,N-binucleophilic reagents, cyclization, exocyclic bondsAbstract
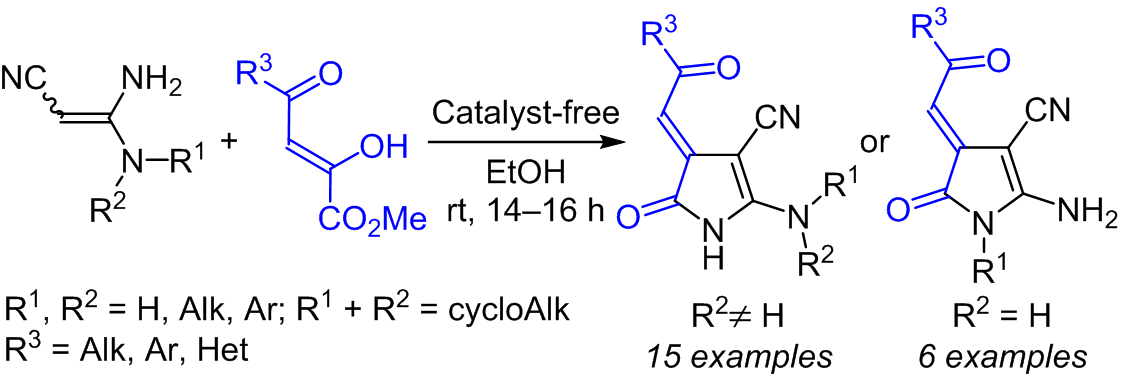
Pyrroles incorporating conjugated exocyclic C=C and C=O bonds were obtained in the reaction of 3,3-diaminoacrylonitriles with aroyl(heteroaroyl)pyruvic acid esters. A simple and efficient method for the synthesis of pyrroles was developed based on this reaction, which makes it possible to vary the substituents in positions 1 and 5 of the pyrrole ring and in the 2-oxoethylidene fragment.
Downloads
Additional Files
Published
2024-12-13
Issue
Section
Original Papers
