THE SYNTHESIS OF INDOLIZIN-1-YLPHOSPHONATES <i>VIA</i> THE REACTION OF ETHYNYLPHOSPHONATES WITH PYRIDINIUM METHYLIDES
Keywords:
ethynylphosphonates, indolizine, indolizin-1-ylphosphonates, pyridinium methylides, [3+2] cycloadditionAbstract
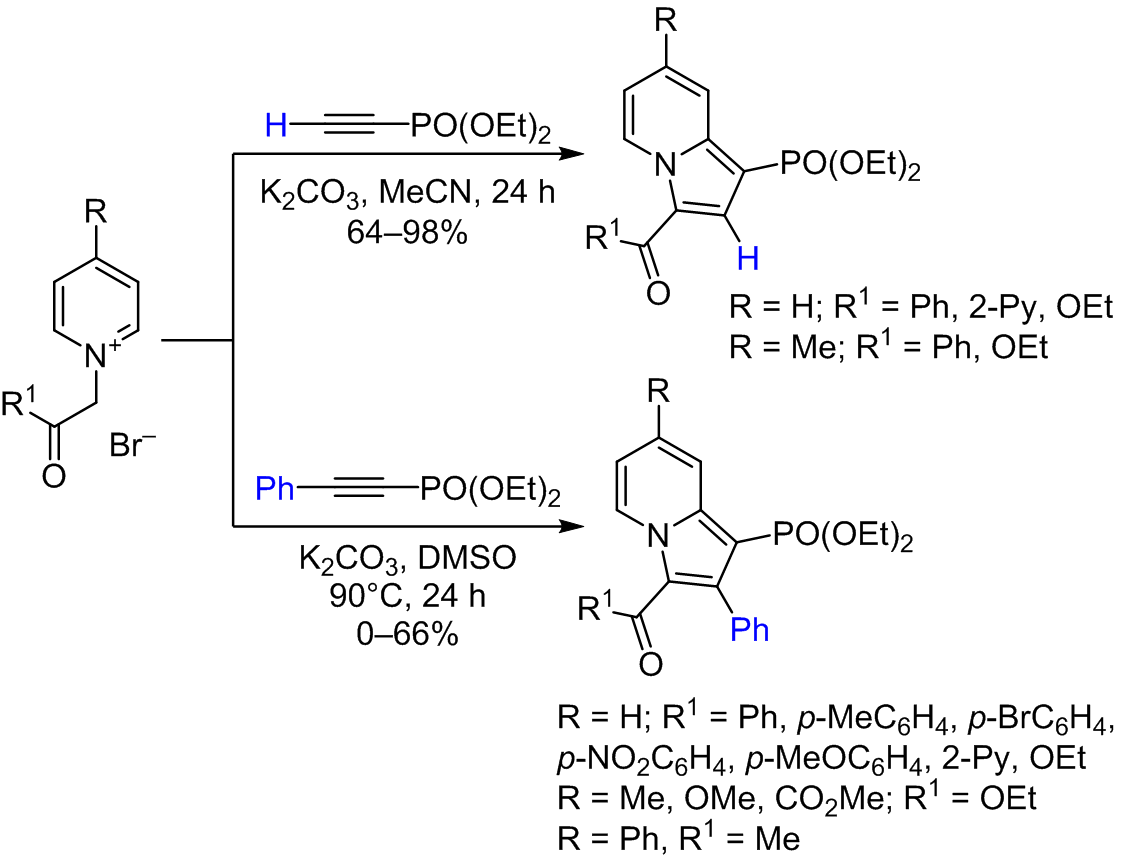
The reaction of diethyl ethynyl- and 2-phenylethynylphosphonates with pyridinium methylides generated in situ from phenacyl- and 2-ethoxy-2-oxoethylpyridinium salts by the action of a base was studied. As a result of the reaction, the corresponding diethyl indolizin-1-ylphosphonates were formed. In this case, diethyl ethynylphosphonate demonstrated higher reactivity and underwent a reaction at room temperature in the K2CO3–MeCN system, while reactions with 2-phenylethynylphosphonate proceeded at elevated temperatures and resulted in lower indolizine yields.