3-SPIROANDROSTENE-SUBSTITUTED 1,3,4-THIADIAZOLINES
Keywords:
androstene, oxamic acid thiohydrazides, spiro-1,3,4-thiadiazolines, steroidal 3-ketonesAbstract
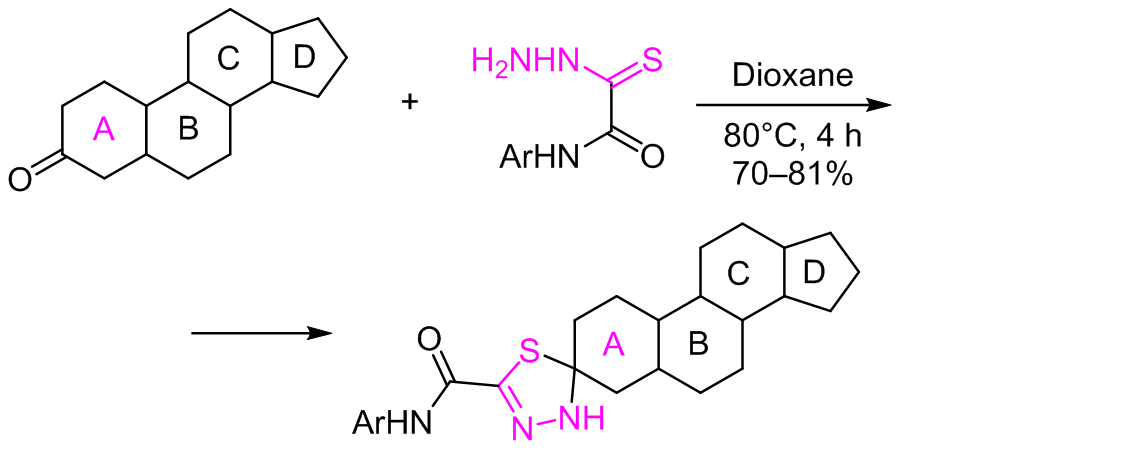
A method for the synthesis of 1,3,4-thiadiazoline spiro steroids via ring A by the reaction of steroid 3-ketones with oxamic acid thiohydrazides was developed. It was shown that if a keto group was present both in ring A and in ring D of the precursor steroid, the reaction occurred only at the keto group of ring A. A number of new steroidal 3-spiroandrostene-substituted 1,3,4-thiadiazolines were obtained, which could be easily acetylated at the thiadiazole NH group.
Downloads
Additional Files
Published
2024-05-28
Issue
Section
Original Papers
