THE SYNTHESIS OF 3-AROYL-1-СARBOXAMIDE-SUBSTITUTED INDOLIZINES
Keywords:
indolizines, pyridinium salt, pyrimidines, cyclization reaction, SNVin reactions, stereoselectivity, Thorpe reactionAbstract
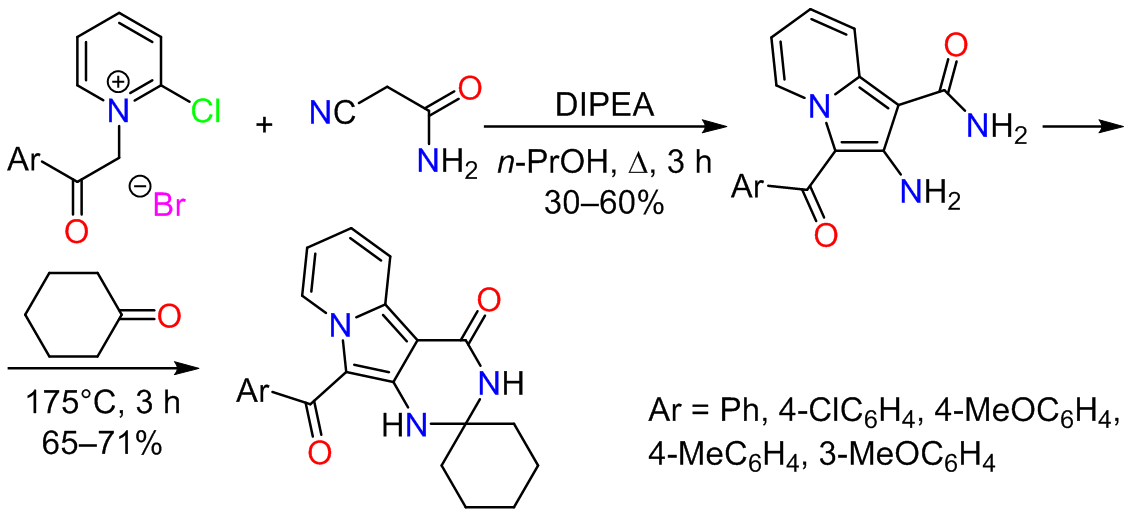
This study reports the synthesis of pyridinium salts achieved by heating 2-chloropyridine with substituted bromoacetophenones in the absence of solvent. The synthesized salt exhibits a high reactivity toward nucleophilic substitution reactions with C-nucleophiles derived from acetonitrile. Furthermore, the resulting salts are efficiently transformed into 2-amino-3-aroylindolizine-1-carboxamides via a cyclization process, leading to a high yield of the desired product. These findings highlight the potential of this synthetic route to produce valuable indolizine derivatives.
Downloads
Additional Files
Published
2025-09-18
Issue
Section
Short Communications
