CONFORMATIONALLY RESTRICTED HYDANTOINS DERIVED FROM BRIDGEHEAD FUNCTIONALIZED CAMPHORQUINONES
Ключевые слова:
hydantoin, α-diketone, Grob fragmentation, Bucherer-Bergs reaction, camphorАннотация
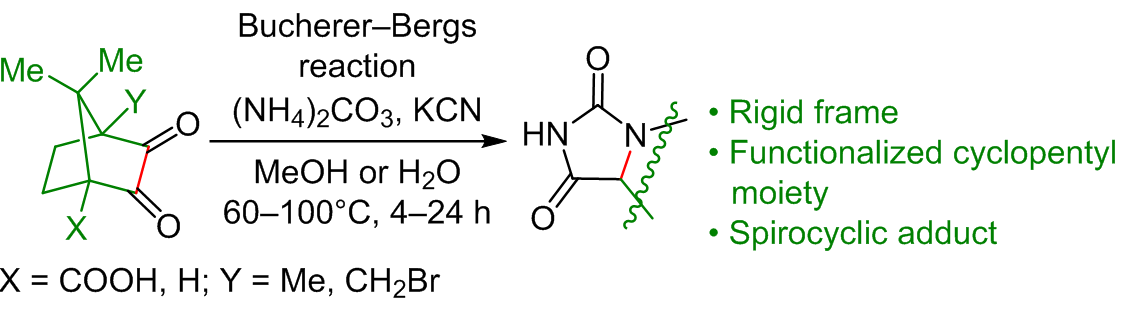
Regio- and stereoselective cleavage of derived camphor α-diketones bearing functional group at the bridgehead position under Bucherer–Bergs reaction conditions was studied for a range of model compounds that are easily accessible from naturally occurring feedstock. In all cases, cleavage of the carbon–carbon bond between the carbonyl groups led to the formation of functionalized hydantoins containing cyclopentane moiety in their structures. Products were obtained in high yields with no purification required, warranting the use of this methodology for the development of libraries of biorelevant compounds.
Загрузки
Опубликован
2024-01-10
Выпуск
Раздел
Оригинальные статьи
