PSEUDOPEPTIDES BASED ON NICOTINIC ACID WITH 4-AMIDOXIME UNIT
Ключевые слова:
4-cyanonicotinic acid, Pyrrolidine ring opening, Amidoximes, methyl 2-(1-imino-3-oxo-1,3-dihydro-2H-pyrrolo[3,4-c]pyridin-2-yl)alkanoates, monoimino derivatives of pyrrolo[3,4-c]pyridine-1,3(2H)-dione, pseudopeptidesАннотация
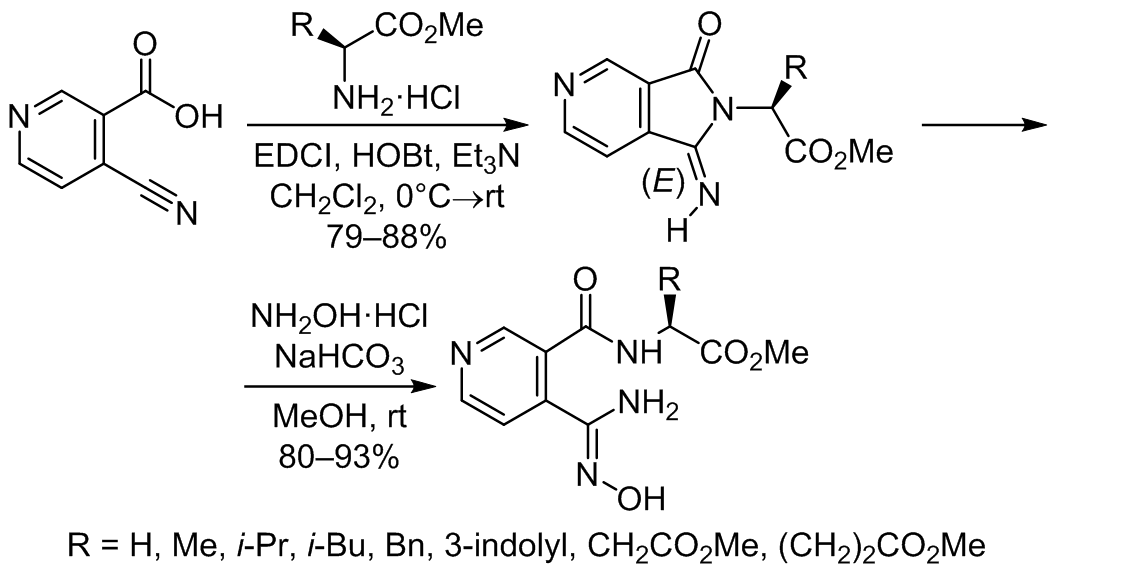
The carbodiimide-mediated coupling of 4-cyanonicotinic acid with L-amino acid methyl esters takes place with the predominant formation of bicyclic compounds – methyl 2-(1-imino-3-oxo-1,3-dihydro-2H-pyrrolo[3,4-c]pyridin-2-yl)alkanoates, which are monoimino derivatives of 4-azaphthalimide. Under the action of hydroxylamine they undergo pyrrolidine cycle opening with the formation of pseudopeptides – coupling products of nicotinic acid with amino acid esters bearing an amidoxime function at position 4.
Загрузки
Опубликован
2024-01-10
Выпуск
Раздел
Оригинальные статьи
