RING-CHAIN ISOMERISM OF ALDEHYDE 2-ALKYLSEMICARBAZONES: EXPERIMENTAL AND THEORETICAL STUDIES. NOVEL SEMICARBAZONE-BASED SYNTHESIS OF 2-ALKYL-2,4-DIHYDRO-3<i>H</i>-1,2,4-TRIAZOL-3-ONES
Keywords:
aldehyde semicarbazones, ring-chain isomerism, oxidative aromatization, DFT calculations, 2,4-dihydro-3H-1,2,4-triazol-3-ones, 1,2,4-triazolidin-3-onesAbstract
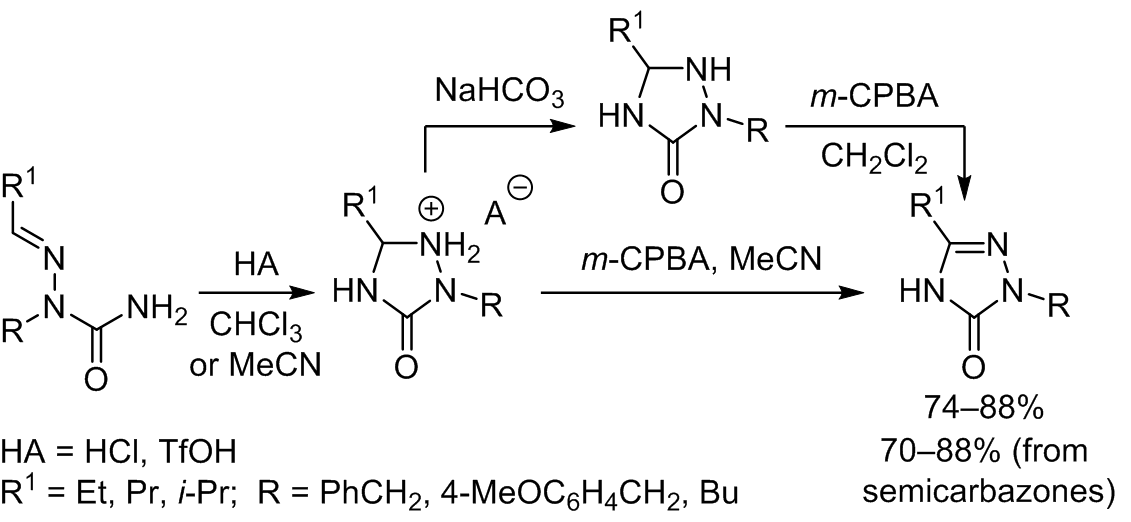
Ring-chain isomerism of aldehyde semicarbazones/1,2,4-triazolidin-3-ones was first studied in detail. Aliphatic aldehyde semicarbazones completely cyclized under the action of very strong Brønsted acids (TfOH, HCl) in aprotic solvents at room temperature to give the corresponding salts of the N1-protonated 1,2,4-triazolidin-3-ones. Both the obtained salts and their free bases are unstable in the presence of air oxygen and undergo slow oxidative aromatization. A novel synthesis of 2-alkyl-2,4-dihydro-3H-1,2,4-triazol-3-ones via the TfOH-promoted cyclization of aliphatic aldehyde semicarbazones followed by aromatization of the isolated crude 1,2,4-triazolidin-3-ones or their hydrotriflates with m-CPBA was developed. In contrast to aliphatic aldehyde semicarbazones, the cyclization of benzaldehyde semicarbazones does not proceed in the presence of strong Brønsted acids. Thermodynamic and kinetic characteristics of the aldehyde semicarbazone ring-chain isomerism are discussed based on the DFT B3LYP/6-311++G(d,p) calculations.