Synthesis of bis(het)aryl systems <i>via</i> domino reaction involving (2<i>E</i>,4<i>E</i>)-2,5-dinitrohexa-2,4-diene: DFT mechanistic considerations
Аннотация
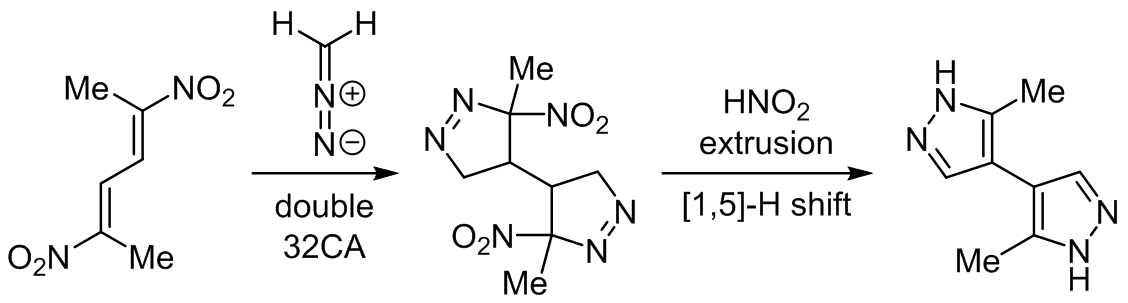
The molecular mechanism of reaction between (2E,4E)-2,5-dinitrohexa-2,4-diene and diazomethane to synthesize 3(5),3'(5')-dimethyl-4,4'-bispyrazole was examined within the molecular electron density theory at the ωB97XD/6-311G(d,p) and B3LYP/6-31G(d) theory levels. Topological analysis of the electron localization function confirms the conjugated character of nitrodiene as well as indicates the allenic pseudoradical electronic structure of diazomethane. Analysis of the reactivity for presented reactions suggests that nitrodiene as well as both isomeric nitrovinyl pyrazolines will participate as electrophiles, while diazomethane will play the role of nucleophilic agent. In turn, both of kinetic and thermodynamic aspects as well as analysis of all critical structures indicate that the formation of bispyrazoline in double cycloaddition is realized via one-step polar asynchronous mechanism. In turn, the conversion of bispyrazoline to bispyrazol occurs via sequence of competitive reactions of one-step HNO2 elimination and one-step [1,5]-H shift. Both of these transformations are performed via nonpolar asynchronous one-step mechanisms, without ionic intermediates.