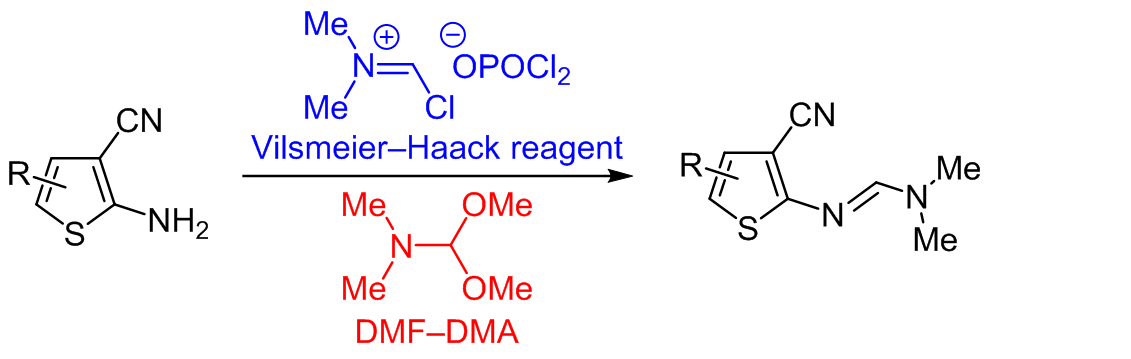
COMPARATIVE STUDY OF VILSMEIER–HAACK REAGENT AND DMF–DMA IN THE SYNTHESIS OF 2-AMINOTHIOPHENE-BASED FORMAMIDINE DERIVATIVES
Ключевые слова:
2-aminothiophene, formamidine derivatives, heterocyclic compounds, DFT study, molecular electrostatic potentials, single crystalАннотация
For the first time, a comparative study has been carried out between Vilsmeier–Haack reagent and N,N-dimethylformamide dimethyl acetal (DMF–DMA) for the preparation of new formamidine derivatives from substituted primary amines. Formamidine derivatives are very useful precursors giving access to a large number of active substituted heterocyclic compounds. The comparison of time, temperature, and yield between these two reagents for the synthesis of N,N-dimethylformamidine derivatives shows a preference for the Vilsmeier–Haack reagent. Two reagents under study have been investigated theoretically and it has been revealed that the Vilsmeier–Haack reagent is more thermodynamically favorable than DMF–DMA. These results provide a guide to chemists in choosing the appropriate reagent when preparing formamidine derivatives. The new products have been structurally confirmed by spectroscopic data and single crystal X-ray diffraction.