SYNTHESIS AND HDAC INHIBITORY ACTIVITY OF PYRIMIDINE-BASED AMIDOXIMES, 1,2,4-OXADIAZOLES, AND 1,2,3,5-OXATHIADIAZOLES
Ключевые слова:
amidoximes, nitrogen heterocycles, 1,2,4-oxadiazoles, 1,2,3,5-oxathiadiazoles, non-hydroxamate HDAC inhibitorsАннотация
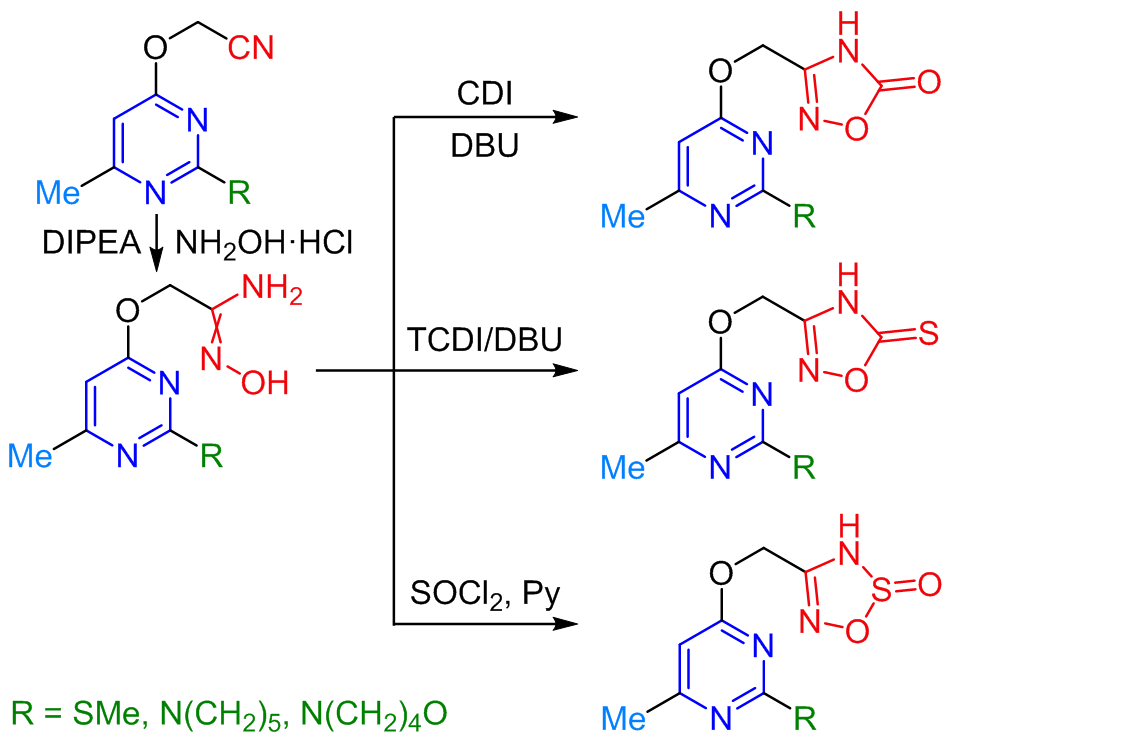
A series of new pyrimidine-based amidoximes, 1,2,4-oxadiazoles, and 1,2,3,5-oxathiadiazoles were synthesized by sequential O-alkylation of 2-substituted 6-methylpyrimidin-4(3H)-ones with chloroacetonitrile followed by the reaction of the obtained nitriles with hydroxylamine to give the corresponding amidoximes and their cyclization under the treatment with 1,1'-carbonyldiimidazole, 1,1'-thiocarbonyldiimidazole, or thionyl chloride into 1,2,4-oxadiazol-5(4H)-ones, 1,2,4-oxadiazole-5(4H)-thiones, or 3H-1,2,3,5-oxathiadiazole 2-oxides, respectively. Study of the inhibitory activity of 15 newly synthesized compounds against the human hystone deacetylase HDAC4 and HDAC8 revealed that, out of the tested series, only N'-hydroxy-2-[6-methyl-2-(piperidin-1-yl)pyrimidin-4-yloxy]acetimidamide moderately inhibited HDAC8 (IC50 5 μM).