STEREOCHEMICAL ASPECT OF A BIGINELLI-LIKE REACTION INVOLVING CYCLOHEXANONE AND 3-AMINO-1<i>H</i>-1,2,4-TRIAZOLE
Ключевые слова:
multicomponent reaction, microwave irradiation, selectivity, stereoselectivity, Biginelli condensation, diastereomerАннотация
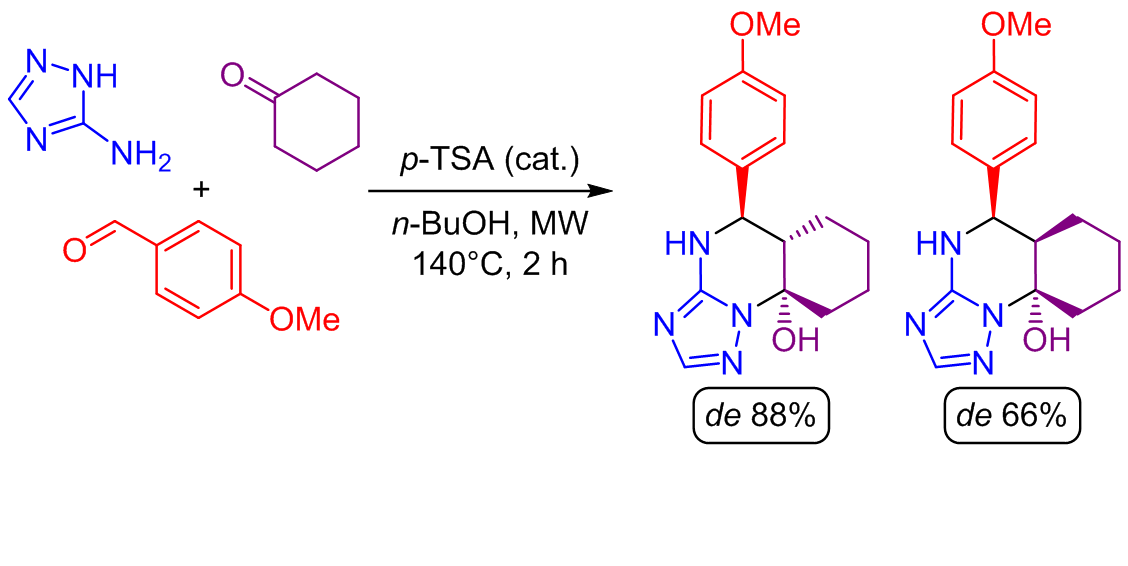
Previous studies of the multicomponent reaction using acetone or more complicated ketones with 3-amino-1H-1,2,4-triazole and benzaldehydes opened the door for diastereoselective formation of 4,5,6,7-tetrahydro[1,2,4]triazolo[1,5- a]pyrimidine derivatives as an alternative product type in Biginelli-like condensations. In this work, replacing acetone with cyclohexanone in this transformation led to the formation of two diastereomeric hexahydro[1,2,4]triazolo[1,5-a]quinazolin-9a(4H)-ols formed in 2:1 ratio, which were isolated with diastereomeric excess of 88 and 66%, correspondingly. The reaction proceeds in a one-pot manner at 140°C for 2 h in n-BuOH under microwave irradiation in the presence of p-toluenesulfonic acid as a catalyst. Both diastereomers were characterized using 2D NMR, with full assignment of proton and carbon signals, and further confirmed by X-ray crystallography. Characteristic proton signals for each diastereomer were identified and a plausible mechanism of reaction was proposed based on the stereochemical structures of the products and literature data.